Which Statement Describes the Bonds in Nitrate
A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it. Has one atom 5____Using electronegativity determine which of the following bonds is the most polar.

Solved Which Of These Statements Describe The Bonding In The Chegg Com
B Fe and S have a covalent bond and S and O have covalent bonds too.

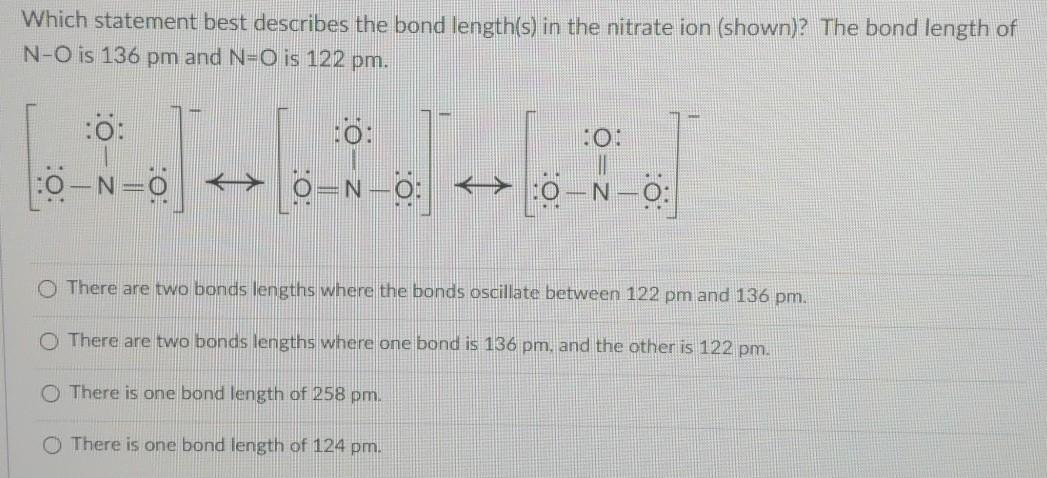
. D X is harder and stronger than Y. A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it. The bond length of N-O is 136 pm and NO is 122 pm.
Ammonium nitrate is added to water at 25 C and the mixture stirred. Use the table to answer the question. Monomers have a CC double bond.
There are three o bonds one is formed by the overlap of sp2 hybrid orbitals on N O. Which one of the following statements best describes the enthalpy change of a reaction. B Nitrite has longer and weaker bonds than nitrate.
Dur to resonance none of the cannonical structures explain the bond length in nitrate except the resonance hybrid which happens to be the last drawn structure. Up to 24 cash back 22Magnesium nitrate contains chemical bonds that are ACuO BCuO2 CCu2O DCu2O2 23Which formula represents copperI oxide. Bond bond energy in.
Which of the following statements correctly describe bond energy. B Nitrogen and oxygen have an electronegativity difference of 05 so the bond is nonpolar covalent. A Chlorine is formed when it is heated with ammonium chloride.
Lewis diagrams for the nitrate and nitrite ions are shown below. C The energy released by bond breaking is greater than the energy absorbed for bond formation. Because Iron is transition metal while S is a non-metal.
A Nitrogen and oxygen have an electronegativity difference of 05 so the bond is polar covalent with oxygen pulling the electrons toward it. NO3-nitrate PO43-phosphate SO42-sulfate SO32-sulfite. Choose the statement that correctly describes the relationship between the two ions in terms of bond length and bond energy.
The energy consumed when chemical bonds are broken during a chemical reaction C. Nitrite has longer and stronger bonds than nitrate. Select all that apply There is one o bond formed by the overlap of two p orbitals.
Hreactants Hproducts D. B Nitrogen and oxygen have an electronegativity difference of 05 so the bond is nonpolar covalent. This means that you can write Bond 13 1 13 1 13 2 23 23 133 The bond between nitrogen and oxygen in the nitrate anion is somewhere between a single bond and a double bond but closer to a single bond.
C Cl 6____Determine the approximate electronegativity difference and identity polar non-polar of the bond between carbon and fluorine. Compound heated gases. The bonds prevent electrons from moving throughout the crystal so a solid ionic compound is a poor conductor.
Which statement best describes the bond length s in the nitrate ion shown. Which of these statements describe the bonding in the nitrate ion. B The energy absorbed for bond breaking is less than the energy released by bond formation.
24 A student heated the carbonates and nitrates of sodium and copper. The energy released when chemical bonds are formed during a chemical reaction B. Nitrite has shorter and stronger bonds than nitrate.
Which statement describes what happens in this reaction. C It reacts with an acid to produce a salt and water. A Methane has weaker covalent bonds than water.
Which statement describes the bonds in nitrate NO3-. Lewis diagrams for the nitrate and nitrite ions are shown below. Two are formed by overlap of an sp3 hybrid orbital on N an sp2 hybrid orbital on O.
The statement that describes the bonds in FeSO₄ is. Aelectrovalent Bionic Cnonpolar covalent Dpolar covalent 24Two atoms of element A unite to form a molecule with the formula A2. B It turns Universal Indicator green.
The bonds prevent ions from moving throughout the crystal so a solid ionic compound is a poor conductor. A X is a pure metal and Y is a. Which statement describes a reaction of potassium hydroxide.
The difference between the energy released by. The results are shown. Describe the bonding in ethylene C2H4 in terms of valence bond theory.
Has a double bond e. The pi-bond electrons are delocalized over the three N-O bonds. The bonds strongly hold ions together reducing the boiling point.
Image is not available to copy A Nitrite has longer and stronger bonds than nitrate. The main reason for your answer being wrong is the phenomenon of resonance in nitrate ion dur to presence of a conjugated system. The bond that forms between a Fe iron and S sulfur is a covalent bond.
All of the above are true. Covalent bonds are also found in the bonding of S and O because they are both Non-metals. 19 Which statement describes a weak acid.
The resonance is shown in the figure below. The bonds weakly hold ions together increasing the melting point. Group 1 elements have an average electronegativity of 084 not including hydrogen.
There are two bonds lengths where the bonds oscillate between 122 pm and 136 pm. Choose the statement that correctly describes the relationship between the two ions in terms of bond length and bond energy. Describe the bonding in ethylene C2H4 in terms of valence bond theory.
Metroplex Corporation will pay a 304 per share dividend next year. The bond energies are shown in the table. The bond between the atoms in the molecule is AH Br BH Cl CI Br DI.
Chemistry questions and answers. Which statement describes the bonds in nitrate NO3-. D sodium nitrate 19 Potassium hydroxide is a base.
Hreactants Hproducts 3. Bond energy values are always positive. The total energy required to break apart a molecule depends on the number and type of bonds.
Chemistry 26102021 1650 SkinnestXOXO. Which statement correctly describes X and Y. There are two bonds lengths where one bond is 136 pm and the other is 122 pm.
Sodium nitrate Which row describes the decomposition products formed when sodium nitrate is heated strongly. 2HBr H2 Br2 Hydrogen. A It is a proton acceptor and is fully ionised in aqueous solution.
Describe the bonding in the nitrate ion NO3- in terms of delocalized molecular orbitals. A The energy absorbed for bond breaking is greater than the energy released by bond formation. 12 Which statement describes an exothermic reaction.
This is called electron delocalization. Nitrite has longer and weaker bonds than nitrate. C X is a solid and Y is a liquid.
Bond Bond Energy HH 432 BrBr 193 II 149 HBr 363 HI 295 Hydrogen bromide breaks down into diatomic hydrogen and bromine via the following reaction. C Nitrogen and oxygen have an electronegativity difference. Correct answer to the question Which statement describes the bonds in nitrate NO3-.

Che 140 Ch 8 Learn Smart Flashcards Quizlet
Solved Which Statement Best Describes The Bond Length S In Chegg Com

Which Statement Describes The Bonds In Nitrate No3 A Nitrogen And Oxygen Have An Brainly Com
No comments for "Which Statement Describes the Bonds in Nitrate"
Post a Comment